Ozempic - Friend or Fad?
April 6, 2023
Ozempic is all the rage these days. The weight loss drug, made famous by the Kardashians and the Red (or, in this year's case, Champagne) Carpet, is now a global phenomenon fueled by the elusive desire for the Miracle Pill that allows us to eat what we want while helping us lose weight. What's not to love? On the surface, it's hard to compete with that. After all, who really likes to change behavior?
What is Ozempic, and how does it work? Here comes the science…
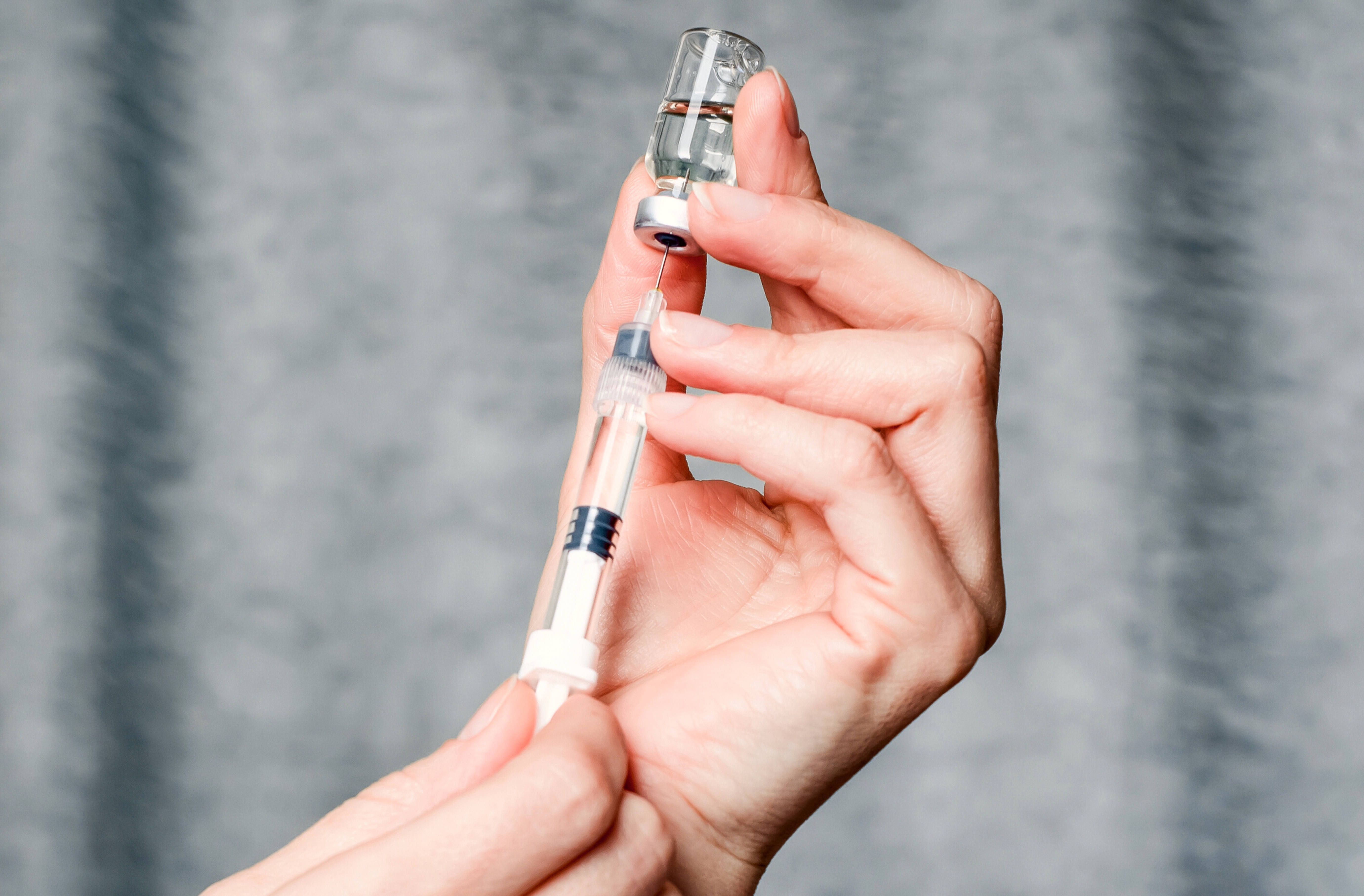
Ozempic has been around for several years. It is a drug initially developed for diabetes. Ozempic is the brand name for semaglutide, a GLP-1 (glucagon-like peptide-1) receptor agonist. GLP-1 is a naturally occurring hormone in the intestines in response to food intake, and it plays an essential role in regulating blood sugar levels in the body. GLP-1 stimulates the production and release of insulin from the pancreas, which helps to lower blood sugar levels after meals. It also reduces the production of glucagon, another hormone that raises blood sugar levels and slows down the emptying of the stomach to help prevent spikes and crashes in blood sugar levels.
Ozempic is an injection typically administered weekly. It works by binding to and activating GLP-1 receptors in the body, which leads to increased insulin production, decreased glucagon secretion, and reduced liver glucose production. This results in lower blood sugar levels and improved glycemic control in people with type 2 diabetes. It also brings more satiety (and initially a feeling of nausea), so people have less desire to eat. More recently, the FDA approved semaglutide in a higher dosage for obesity. That drug is known by its brand name, Wegovy.
What results can I expect from Ozempic? And what happens when I stop?
People claim that the drug Ozempic is causing them to shed pounds fast. While it’s true that both medications can make you lose weight, it may not be time to run to the pharmacy just yet.
What has caused the big stir is that studies (and celebrities) have shown that people taking Ozempic, on average, lose about 10% - 15% of their body weight over 12 months (1, 2). Side effects of taking Ozempic and its stronger cousin, Wegovy, include nausea and vomiting, diarrhea, constipation, headache, dizziness, pancreatitis (pancreas inflammation), muscle pain or weakness, and trouble sleeping (insomnia).
Along with the weight loss (hurray), however, comes an average of ten beats per minute increase in resting heart rate. Why does that matter, you ask? While we are unsure about the exact mechanism causing this increase, the resultant increase in resting heart rate should give moment to pause. A report in the Canadian Medical Journal and others (3, 4, 5) suggests that for every 10 beats per minute increase in resting heart rate, the risk of dying from any cause rose 9 percent, and the risk of dying from heart disease increased 8 percent.
Furthermore, the observed weight loss is dominated by muscle mass loss rather than fat loss (6). For example, for every 10 pounds of weight loss, typically people lose more than 6 pounds of muscle mass for every 4 pounds of fat loss. Loss of muscle mass, especially as we age, is detrimental to our metabolic health (7).
There is clear data on what happens when people stop taking these drugs. One of the challenges of weight loss is maintaining that loss over extended time. All of the studies showed that weight loss was reversed once medication was stopped. The amount that was regained was dependent upon how much was initially lost. Those that lost about 5% of their weight initially put it all back on and some. They were actually above their starting weight one year after coming off the medication. Those that lost between 5-15% went pretty much back to their starting weight. Whereas those that initially lost 20% regained about 12% of their weight loss. So sustained weight loss is really only observed in those that lost considerable weight (2).
In contrast, Plantable's clinical results and underscored in its clinical trials, people experience 5% weight loss in the first month and then lose 10% of their initial body weight within the first three to six months. The immediate and significant weight loss in that first Reboot month is coupled with a steady 1-1½ pounds per week after that until that healthy equilibrium weight is reached, with early clinical trial indications showing that weight loss is maintained at the six- and 12- month marker. Furthermore, our clinical trials have indicated that the balance of fat loss versus muscle mass loss is skewed towards fat loss rather than dominated by muscle loss, rather than the opposite that is observed with semaglutide.
The Bottom Line
Along with the similar, even newer entrant to the market, tirzepatide (known as Mounjaro), these drugs do work for weight loss. They alter hormonal function to lower blood sugar while promoting satiety, improving blood sugar control for those with diabetes, and weight loss. While a shift to a healthier BMI is a health improvement for sure, the proportional increase in resting heart rate coupled with extreme muscle mass loss is not a positive contributor to metabolic health.
The cost of these drugs is about $1,000 per month. The FDA approves these drugs for treating diabetes and obesity and, as a result, are reimbursed by many insurance companies (though they are not authorized to help get you back into your skinny jeans). A 12-month supply will set you, your insurance company, and/or the taxpayer back $12K, with no lasting effect once taking the drug is ceased, unless we change our behavior. It's an expensive short-term "luxury" which frankly may even be causing us metabolic harm unless we are significantly overweight, and this is the last straw.
Our approach at Plantable has always been to tackle the Root Issue. While it is hard to compete with the ever-so-tempting Miracle Drug, the results achieved with Plantable are superior to those achieved by Ozempic, coupled with an undeniable improvement in metabolic health, with long-lasting results. Modestly, we think that is a miracle 💚. It's time for the insurance companies to wake up!
1. Wadden, T. A., Bailey, T. S., Billings, L. K., Davies, M., Frias, J. P., Koroleva, A., Lingvay, I., O’Neil, P. M., Rubino, D. M., Skovgaard, D., Wallenstein, S. O. R., Garvey, W. T., & STEP 3 Investigators. (2021). Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA, 325(14), 1403–1413. https://doi.org/10.1001/jama.2021.1831
2. Wilding, J. P. H., Batterham, R. L., Calanna, S., Davies, M., Van Gaal, L. F., Lingvay, I., McGowan, B. M., Rosenstock, J., Tran, M. T. D., Wadden, T. A., Wharton, S., Yokote, K., Zeuthen, N., Kushner, R. F., & STEP 1 Study Group. (2021). Once-Weekly Semaglutide in Adults with Overweight or Obesity. The New England Journal of Medicine, 384(11). https://doi.org/10.1056/NEJMoa2032183
3. Zhang, D., Shen, X., & Qi, X. (2015). Resting heart rate and all-cause and cardiovascular mortality in the general population: a meta-analysis. Canadian Medical Association Journal, 188(3), E53–E63. https://doi.org/10.1503/cmaj.150535
4. Saxena, A., Minton, D., Lee, D., Sui, X., Fayad, R., Lavie, C. J., & Blair, S. N. (2013). Protective Role of Resting Heart Rate on All-Cause and Cardiovascular Disease Mortality. Mayo Clinic Proceedings, 88(12), 1420–1426. https://doi.org/10.1016/j.mayocp.2013.09.011
5. Woodward, M., Webster, R., Murakami, Y., Barzi, F., Lam, T.-H., Fang, X., Suh, I., Batty, G. D., Huxley, R., & Rodgers, A. (2012). The association between resting heart rate, cardiovascular disease and mortality: evidence from 112,680 men and women in 12 cohorts. European Journal of Preventive Cardiology, 21(6), 719–726. https://doi.org/10.1177/2047487312452501
6. Sargeant, J. A., Henson, J., King, J. A., Yates, T., Khunti, K., & Davies, M. J. (2019). A Review of the Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors on Lean Body Mass in Humans. Endocrinology and Metabolism, 34(3), 247. https://doi.org/10.3803/enm.2019.34.3.247
7. McCrimmon, R. J., Catarig, A.-M., Frias, J. P., Lausvig, N. L., le Roux, C. W., Thielke, D., & Lingvay, I. (2020). Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia, 63(3), 473–485. https://doi.org/10.1007/s00125-019-05065-8

